Abstract
Background Among the different scenarios of pregnancy and chronic myeloid leukemia (CML) the situation in which CML is diagnosed during pregnancy is one of the most challenging for management. The high survival rate of patients (pts) in chronic phase (CP) encourages support of continuation of the pregnancy, but there is a lack of information regarding optimal therapy and weighting the risks/benefits for both mother and baby remains difficult.
Aim To describe therapy and outcomes in pts with CML at onset during pregnancy.
Methods Data were obtained both retrospectively and prospectively from 11 countries through the ELN CML pregnancy registry since 2001. Clinical and demographic data, therapy, pregnancy outcomes and follow-up were analyzed.
Results We have data from a total of 400 pregnancies in 298 CML women (including consecutive pregnancies) as of June 2022. CML was diagnosed during pregnancy in 86/298 (29%) pts, all in CP.
Median (Me) age was 27 years (range 18-41). CML was diagnosed during 1st, 2nd and 3rd trimester (trim) in 49(57%), 21(24%) and 16(19%) pts, respectively, with Me CML onset at 11 weeks gestation (range 5-38). Sokal score was low, intermediate and high in 61(71%), 15(17%) and 5(6%) respectively; not available in 5(6%). Sixty-five (76%) pregnancies ended in childbirth, 18(21%) elective and 3(3%) spontaneous abortion.
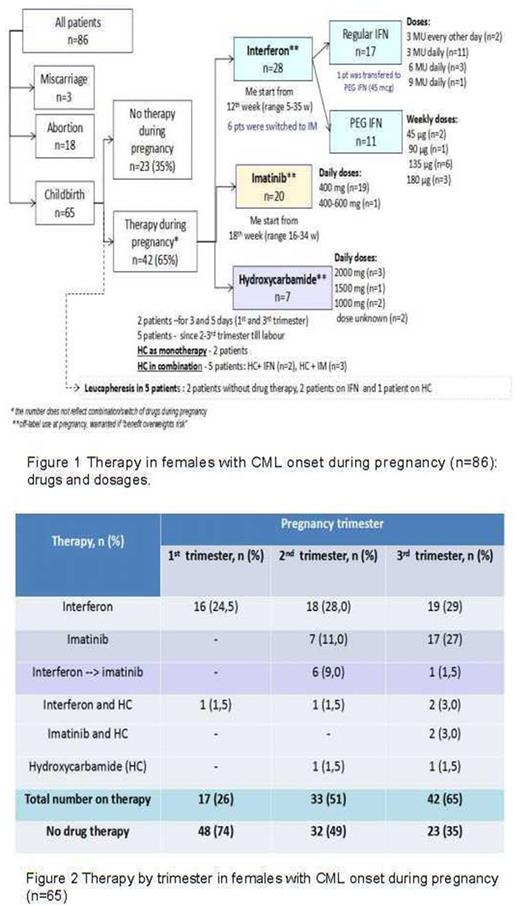
During pregnancy 23(35%) pts were not treated with anti-leukemic drugs , while 42 (65%) did receive therapy (figure 1,2).
In total 28 (43%) pts received interferon (IFN) with Me start of 12 w (range 5-35): 11/28 pts got pegylated IFN (PEG IFN), 16/28 pts received regular (daily dose) IFN and 1 pt got regular IFN in 1st trim and PEG IFN since 2nd trim. Twenty (31%) pts received imatinib (IM) at late pregnancy: Me start was 18 w (range 16-34). Six pts were switched from IFN to IM: 5 in 2nd trim and 1 in 3rd trim. Seven (11%) pts received hydroxycarbamide (HC) as monotherapy or in combination with IFN or IM. Additionally, 5(8%) pts received leucapheresis.
Sixty six children were born (one set of twins). No congenital abnormalities were reported in 64 (97%). One baby had an abdominal skin angioma (exposed to IFN in 2-3 trim and HU in 3rd trim). One baby born at 35 w had a patent foramen ovale (IM since 33w).
Seven newborns were of low birth weight (<2500 g), 2 with no drug exposure, and 2 were exposed to IM, PEG-IFN (n=1), IFN and IM (n=1), HC (n=1), Follow-up of the children was uneventful.
All 86 females received TKIs after pregnancy end: IM 70(81%)/ 2ndgeneration TKI 16(19%). With Me follow-up of 36 months (mo) (range 1 mo-17 years) 23/70(33%) pts on IM were switched to other TKI.
The best overall response was at least major molecular response in 65(86%) of 75 evaluable pts with a treatment period >6 mo.
No CML progression was observed during pregnancy. However, 3 pts unfortunately died 19, 24 and 119 mo after pregnancy completion, the reasons were not thought to be related to management in pregnancy: non-compliance, progression/resistance (1 case with T315/F359 mutation in the time of pre-ponatinib availability) and death after bone marrow transplantation.
Conclusions More than 20 years of international efforts to collect data on pregnancy in women with CML can conclude that there is a real possibility of normal childbirth in women diagnosed with CP CML during pregnancy. We did not observe an increased rate of birth abnormalities nor life threatening events including cases treated with IM at late pregnancy. It is reasonable to balance the risks for both the safety of mother and infant and the efficacy of treatment. IFN can be used from 1st trim and may be prolonged in cases of good disease control and good tolerability. IM is probably safe in 2nd- 3rd trim considering its limited placental crossing. The use of HC is more questionable, as it has no advantages over IM and passes freely to the placenta: its use may be acceptable if there is a need for the urgent cytoreduction and leucapheresis is not available.
Disclosures
Chelysheva:Novartis Pharma: Speakers Bureau; Pfizer: Speakers Bureau. Turkina:Novartis: Other: Travel, Accommodation, Expenses , Speakers Bureau; Pfizer: Other: Travel, Accommodation, Expenses , Speakers Bureau; Fusion Pharma: Speakers Bureau. Apperley:Pfizer: Honoraria, Research Funding, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Rea:Pfizer: Consultancy; Novartis: Consultancy; Incyte: Consultancy. Nicolini:Novartis Services, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sun Pharma Ltd: Consultancy; KARTOS: Consultancy; Incyte biosciences: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees. Kim:Il-Yang: Consultancy, Honoraria, Research Funding, Speakers Bureau; Otsuka: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau. Byrne:Novartis: Honoraria, Speakers Bureau. Marco:jazz: Honoraria; abbvie: Honoraria; janssen: Honoraria; incyte: Membership on an entity's Board of Directors or advisory committees. Abruzzese:BMS, Incyte, Novartis, Pfizer: Consultancy.
OffLabel Disclosure:
off-label use of imatinib, interferon, hydroxiurea during pregnancy in patients with chronic myeloid leukemia
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal